Shows
 MIDI Innovation Vault ™Ep 5 Series 10 | Regionalized Point-of-Care ApproachStep into the captivating world of cutting-edge cancer treatment in this episode unraveling the mysteries of CAR T-cell therapy, exploring its potential to transform the lives of cancer patients and the pivotal role that Point-of-Care (POC) manufacturing plays in making this groundbreaking therapy accessible and affordable to a larger population.
In this technical and engineering-focused episode, Chris Montalbano, MIDI CEO, unveils MIDI's pioneering journey in developing an automated instrument with a contiguous disposable kit for the fabrication of autologous CAR T-cells. Departing from the conventional centralized manufacturing approach, this innovative automated system aims to...2023-08-0311 min
MIDI Innovation Vault ™Ep 5 Series 10 | Regionalized Point-of-Care ApproachStep into the captivating world of cutting-edge cancer treatment in this episode unraveling the mysteries of CAR T-cell therapy, exploring its potential to transform the lives of cancer patients and the pivotal role that Point-of-Care (POC) manufacturing plays in making this groundbreaking therapy accessible and affordable to a larger population.
In this technical and engineering-focused episode, Chris Montalbano, MIDI CEO, unveils MIDI's pioneering journey in developing an automated instrument with a contiguous disposable kit for the fabrication of autologous CAR T-cells. Departing from the conventional centralized manufacturing approach, this innovative automated system aims to...2023-08-0311 min MIDI Innovation Vault ™Ep 4 Series 10 | CAR T-cell Therapy Production & Treatment, Instituting DemocratizationMIDI explores the current landscape of CAR T-cell therapy manufacturing and its impact on patient treatment. MIDI's CEO, Christopher Montalbano, sheds light on the challenges posed by the centralized manufacturing approach and delves into the promising alternative: Point-of-Care manufacturing.
Chris breaks down the centralized manufacturing workflow for autologous CAR T-cell therapy, emphasizing the collection of T-cells from patients, their transportation to a centralized lab for genetic manipulation and cell expansion, and the subsequent infusion back into the patient. While highlighting the benefits of this therapy, he unveils the critical issues arising from the current...2023-07-2412 min
MIDI Innovation Vault ™Ep 4 Series 10 | CAR T-cell Therapy Production & Treatment, Instituting DemocratizationMIDI explores the current landscape of CAR T-cell therapy manufacturing and its impact on patient treatment. MIDI's CEO, Christopher Montalbano, sheds light on the challenges posed by the centralized manufacturing approach and delves into the promising alternative: Point-of-Care manufacturing.
Chris breaks down the centralized manufacturing workflow for autologous CAR T-cell therapy, emphasizing the collection of T-cells from patients, their transportation to a centralized lab for genetic manipulation and cell expansion, and the subsequent infusion back into the patient. While highlighting the benefits of this therapy, he unveils the critical issues arising from the current...2023-07-2412 min MIDI Innovation Vault ™Ep 3 Series 10 | Effectiveness of CAR T-cell TherapyIn this episode, MIDI's CEO, Christopher Montalbano, takes us on an enlightening journey into the world of CAR T-cell therapy, sharing invaluable insights into the effectiveness of CAR T-cell therapy against different types of cancer.
CAR T-cell therapy exhibits greater efficacy in hematological malignancies like leukemia and lymphoma, while its impact on solid tumors is more limited. Christopher explains that the challenging nature of solid tumors, with their embedding in tissue and the presence of a hostile microenvironment, poses significant obstacles for CAR T-cells to penetrate and effectively combat the cancerous cells. Moreover, the...2023-07-1006 min
MIDI Innovation Vault ™Ep 3 Series 10 | Effectiveness of CAR T-cell TherapyIn this episode, MIDI's CEO, Christopher Montalbano, takes us on an enlightening journey into the world of CAR T-cell therapy, sharing invaluable insights into the effectiveness of CAR T-cell therapy against different types of cancer.
CAR T-cell therapy exhibits greater efficacy in hematological malignancies like leukemia and lymphoma, while its impact on solid tumors is more limited. Christopher explains that the challenging nature of solid tumors, with their embedding in tissue and the presence of a hostile microenvironment, poses significant obstacles for CAR T-cells to penetrate and effectively combat the cancerous cells. Moreover, the...2023-07-1006 min MIDI Innovation Vault ™Ep 2 Series 10 | How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/DisadvantagesAfter our Episode 1 overview detailing various alternative types of cancer treatments, it appeared that CAR T-cell therapy is the treatment that looks most promising from multiple viewpoints. In Episode 2, How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/Disadvantages, we learn more about how CAR T-cell therapy works by targeting cancer cells while sparing healthy cells.
Listen to Episode 2, "How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/Disadvantages".2023-06-2015 min
MIDI Innovation Vault ™Ep 2 Series 10 | How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/DisadvantagesAfter our Episode 1 overview detailing various alternative types of cancer treatments, it appeared that CAR T-cell therapy is the treatment that looks most promising from multiple viewpoints. In Episode 2, How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/Disadvantages, we learn more about how CAR T-cell therapy works by targeting cancer cells while sparing healthy cells.
Listen to Episode 2, "How CAR T-cell Therapy is Produced & Achieve Efficacy, with Format Types Advantages/Disadvantages".2023-06-2015 min MIDI Innovation Vault ™Ep 1 Series 10 | NexGen CAR T-cell Therapy; Democratization via Advanced Point-of-Care Production and Applications: How does CAR T-cell Therapy Compare to Alternative Methods of Cancer Treatment?In this podcast series on CAR T-cell Therapy, MIDI’s CEO and Co-Founder, Chris Montalbano, discusses his insight into this exciting and rapidly evolving field in cancer treatment.
The first FDA-approved CAR T-cell biopharmaceutical drug was introduced in 2017. Currently there are approximately 10 approved drugs, and the number in development and FDA approval pipeline is growing exponentially. This therapy is unique because it specifically targets cancer cells while sparing healthy cells, in contrast to other treatment methods that can kill both. The precision of this approach has generated much excitement within the medical community, as it h...2023-06-0511 min
MIDI Innovation Vault ™Ep 1 Series 10 | NexGen CAR T-cell Therapy; Democratization via Advanced Point-of-Care Production and Applications: How does CAR T-cell Therapy Compare to Alternative Methods of Cancer Treatment?In this podcast series on CAR T-cell Therapy, MIDI’s CEO and Co-Founder, Chris Montalbano, discusses his insight into this exciting and rapidly evolving field in cancer treatment.
The first FDA-approved CAR T-cell biopharmaceutical drug was introduced in 2017. Currently there are approximately 10 approved drugs, and the number in development and FDA approval pipeline is growing exponentially. This therapy is unique because it specifically targets cancer cells while sparing healthy cells, in contrast to other treatment methods that can kill both. The precision of this approach has generated much excitement within the medical community, as it h...2023-06-0511 min MIDI Innovation Vault ™Ep 5 Series 9 | INNOVATION ROADMAP™: Stop-3, (conclusion) Commercialization & ImplementationListen to this FINAL episode in the series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In this episode, we're joined by Andrew Martin, Vice President of MIDI. So far in this series, we have covered class identification, planning, requirements and MIDI's design control methods using our MATRIX-ALM tool. Now, in this episode, we cover RISK MANAGEMENT (ISO 14971) utilizing MATRIX to document RISK. Listen in as Andrew dives into everything from Risk analysis to production and post-production activities.
Complete the INNOVATION ROADMAP™ tour with us by listening to t...2023-05-0909 min
MIDI Innovation Vault ™Ep 5 Series 9 | INNOVATION ROADMAP™: Stop-3, (conclusion) Commercialization & ImplementationListen to this FINAL episode in the series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In this episode, we're joined by Andrew Martin, Vice President of MIDI. So far in this series, we have covered class identification, planning, requirements and MIDI's design control methods using our MATRIX-ALM tool. Now, in this episode, we cover RISK MANAGEMENT (ISO 14971) utilizing MATRIX to document RISK. Listen in as Andrew dives into everything from Risk analysis to production and post-production activities.
Complete the INNOVATION ROADMAP™ tour with us by listening to t...2023-05-0909 min MIDI Innovation Vault ™Ep 4 Series 9 | INNOVATION ROADMAP™: Stop-3, Part 1 Commercialization & ImplementationThroughout the series, we have been discussing the INNOVATION ROADMAP™ and its application in Medical Device Development. In previous episodes, we explored the first two stops on this journey, which did not require deployment under FDA-QSR and ISO-13485 Design Controls and Risk Management. These stops allowed us to investigate the market, identify opportunities, and refine them through exploratory technology investigations and interactive MVP breadboarding.
In this episode, we are joined by Wolfgang Huber, Co-Founder of Matrix Requirements, who has created a unique and effective cloud-based tool to support companies in the process we discuss. Fo...2023-04-2417 min
MIDI Innovation Vault ™Ep 4 Series 9 | INNOVATION ROADMAP™: Stop-3, Part 1 Commercialization & ImplementationThroughout the series, we have been discussing the INNOVATION ROADMAP™ and its application in Medical Device Development. In previous episodes, we explored the first two stops on this journey, which did not require deployment under FDA-QSR and ISO-13485 Design Controls and Risk Management. These stops allowed us to investigate the market, identify opportunities, and refine them through exploratory technology investigations and interactive MVP breadboarding.
In this episode, we are joined by Wolfgang Huber, Co-Founder of Matrix Requirements, who has created a unique and effective cloud-based tool to support companies in the process we discuss. Fo...2023-04-2417 min MIDI Innovation Vault ™Ep 3. Series 9 | INNOVATION ROADMAP™: Stop-2, Technology Innovation and the R&D ProcessIn our previous episode, Chris Montalbano provided an overview of the INNOVATION ROADMAP™ as applied to medical device development in addition to describing the 1st Stop, which was Market Exploration & Discovering Opportunities. In this new episode, Principal and Chief Creative Officer, Gregory Montalbano discusses the INNOVATION ROADMAP™ 2nd Stop, which is Technology Innovation and the R&D Process.
As discussed in prior episodes, the FDA-QSR & ISO 13485 guidance recognizes both Stop 1 Market Exploration & Discovering Opportunities, as well as Stop 2 Technology Innovation and the R&D Process, are essential formative exercises for any business to implement whil...2023-04-1111 min
MIDI Innovation Vault ™Ep 3. Series 9 | INNOVATION ROADMAP™: Stop-2, Technology Innovation and the R&D ProcessIn our previous episode, Chris Montalbano provided an overview of the INNOVATION ROADMAP™ as applied to medical device development in addition to describing the 1st Stop, which was Market Exploration & Discovering Opportunities. In this new episode, Principal and Chief Creative Officer, Gregory Montalbano discusses the INNOVATION ROADMAP™ 2nd Stop, which is Technology Innovation and the R&D Process.
As discussed in prior episodes, the FDA-QSR & ISO 13485 guidance recognizes both Stop 1 Market Exploration & Discovering Opportunities, as well as Stop 2 Technology Innovation and the R&D Process, are essential formative exercises for any business to implement whil...2023-04-1111 min MIDI Innovation Vault ™Ep 2. Series 9 | INNOVATION ROADMAP™: Stop-1, Market Exploration: Discovering OpportunitiesThe first episode of this series provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained the three key stops along this journey.
Now, follow along in Episode 2 as we dive into the 1st Stop on the INNOVATION ROADMAP™, which is: Market Exploration & Discovering Opportunities. The FDA-QSR & ISO-13485 guidance recognizes this as a quintessential activity for any business to perform, yet they make it known that their regulatory controls, such as Design Controls & Risk Management, do not have to be performed at this point. They und...2023-03-2710 min
MIDI Innovation Vault ™Ep 2. Series 9 | INNOVATION ROADMAP™: Stop-1, Market Exploration: Discovering OpportunitiesThe first episode of this series provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained the three key stops along this journey.
Now, follow along in Episode 2 as we dive into the 1st Stop on the INNOVATION ROADMAP™, which is: Market Exploration & Discovering Opportunities. The FDA-QSR & ISO-13485 guidance recognizes this as a quintessential activity for any business to perform, yet they make it known that their regulatory controls, such as Design Controls & Risk Management, do not have to be performed at this point. They und...2023-03-2710 min MIDI Innovation Vault ™Ep 1. Series 9 | The INNOVATION ROADMAP™ Explaining the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485Listen to this first episode as we prepare to explain the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485, with the CEO of MIDI Medical Product Development, Chris Montalbano.
In this episode, Chris outlines how to take control of the medical device development process utilizing a DevelopmentDNA™ approach. He begins by explaining a common misperception in the industry that the Medical Regulatory Design Controls and Risk Management (under ISO-13485) are often viewed as a mandate, a process that will inhibit device innovation.
The perception shift to be revealed will involve the...2023-03-1509 min
MIDI Innovation Vault ™Ep 1. Series 9 | The INNOVATION ROADMAP™ Explaining the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485Listen to this first episode as we prepare to explain the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485, with the CEO of MIDI Medical Product Development, Chris Montalbano.
In this episode, Chris outlines how to take control of the medical device development process utilizing a DevelopmentDNA™ approach. He begins by explaining a common misperception in the industry that the Medical Regulatory Design Controls and Risk Management (under ISO-13485) are often viewed as a mandate, a process that will inhibit device innovation.
The perception shift to be revealed will involve the...2023-03-1509 min MIDI Innovation Vault ™Ep. 3 Series 8 | Deep Dive into Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast episode, MIDI Principal Greg Montalbano will discuss the various Point of Care and At Home Diagnostic testing technology applications covering the details of the science and value proposition for detection, market application, as well as global health.
The technology platforms discussed in this episode include a host of Molecular Diagnostics, including:
- CRISPR
- RT-PCR
- LAMP and others
Listen to Episode 3 to learn more about the rapid acceleration and application of Point of Care and At Home diagnostic platforms. The wide adoption of these testing platforms, coupled with...2022-11-2923 min
MIDI Innovation Vault ™Ep. 3 Series 8 | Deep Dive into Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast episode, MIDI Principal Greg Montalbano will discuss the various Point of Care and At Home Diagnostic testing technology applications covering the details of the science and value proposition for detection, market application, as well as global health.
The technology platforms discussed in this episode include a host of Molecular Diagnostics, including:
- CRISPR
- RT-PCR
- LAMP and others
Listen to Episode 3 to learn more about the rapid acceleration and application of Point of Care and At Home diagnostic platforms. The wide adoption of these testing platforms, coupled with...2022-11-2923 min MIDI Innovation Vault ™Ep. 2 Series 8|Deep Dive into Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast, MIDI’s Principal and Co-Founder, Gregory Montalbano, continues sharing insight into the current landscape as well as what is in store for the future related to Point of Care and at-home diagnostic devices. From evolving market trends and technology innovations to key challenges and unmet opportunities for global health, Greg covers it all.
Episode 2 dives specifically into the details of Point of Care & At Home Diagnostic Testing covering :
• Decentralization of Diagnostic Testing & Regulatory Challenges: CLIA
• POC and At Home Testing Data Privacy Challenges
• And Multiplex Testing and Advancing Molecular Diagnostics2022-11-1615 min
MIDI Innovation Vault ™Ep. 2 Series 8|Deep Dive into Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast, MIDI’s Principal and Co-Founder, Gregory Montalbano, continues sharing insight into the current landscape as well as what is in store for the future related to Point of Care and at-home diagnostic devices. From evolving market trends and technology innovations to key challenges and unmet opportunities for global health, Greg covers it all.
Episode 2 dives specifically into the details of Point of Care & At Home Diagnostic Testing covering :
• Decentralization of Diagnostic Testing & Regulatory Challenges: CLIA
• POC and At Home Testing Data Privacy Challenges
• And Multiplex Testing and Advancing Molecular Diagnostics2022-11-1615 min MIDI Innovation Vault ™Ep. 1 Series 8 | The Deep Dive into the Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast series on Point of Care and At Home Diagnostic Testing, MIDI’s Principal and Co-Founder, Gregory Montalbano, discusses his insight into the current landscape as well as what is in store for the future. Series topics will range from evolving market trends and technology innovations as well as key challenges and unmet opportunities for global health as related to the Point of Care and at-home diagnostic devices.
Greg will also showcase current and emerging methods of detection from qPCR to novel CRISPR diagnostic technology whose express purpose is to improve speed, accuracy, quality and ac...2022-11-0124 min
MIDI Innovation Vault ™Ep. 1 Series 8 | The Deep Dive into the Global Democratization of Point of Care & At-Home Diagnostic TestingIn this podcast series on Point of Care and At Home Diagnostic Testing, MIDI’s Principal and Co-Founder, Gregory Montalbano, discusses his insight into the current landscape as well as what is in store for the future. Series topics will range from evolving market trends and technology innovations as well as key challenges and unmet opportunities for global health as related to the Point of Care and at-home diagnostic devices.
Greg will also showcase current and emerging methods of detection from qPCR to novel CRISPR diagnostic technology whose express purpose is to improve speed, accuracy, quality and ac...2022-11-0124 min MIDI Innovation Vault ™Ep. 3 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotThe discussion continues and series concludes with a deep understanding of the importance, and requirements of the world of Digital Therapeutics, also known as DTx as related to the healthcare device industry.
This episode details DTx solutions go-to-market strategies, define DTx digital therapeutics ecosystem and stakeholders, as well as covering DTx solutions give-to-get value exchanges for Patients, Payers, Providers, Pharma & Medtech. In addition to the three common features for digital therapy adoption by consumers and healthcare providers.2022-07-2717 min
MIDI Innovation Vault ™Ep. 3 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotThe discussion continues and series concludes with a deep understanding of the importance, and requirements of the world of Digital Therapeutics, also known as DTx as related to the healthcare device industry.
This episode details DTx solutions go-to-market strategies, define DTx digital therapeutics ecosystem and stakeholders, as well as covering DTx solutions give-to-get value exchanges for Patients, Payers, Providers, Pharma & Medtech. In addition to the three common features for digital therapy adoption by consumers and healthcare providers.2022-07-2717 min MIDI Innovation Vault ™Ep. 2 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotWe continue the series topic, Digital Therapeutics known as medical treatments that can make medical claims, and receive regulatory authorization and reimbursement.
In this episode, MIDI Principal, Greg Montalbano will dive into the details of Digital Therapeutics covering:
• What is driving demand for Digital Therapeutics
• Establishing Digital Therapeutic Regulatory Quality in today's crowded market
• The forms that Digital Therapeutics take on to serve the medical market
2022-07-1317 min
MIDI Innovation Vault ™Ep. 2 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotWe continue the series topic, Digital Therapeutics known as medical treatments that can make medical claims, and receive regulatory authorization and reimbursement.
In this episode, MIDI Principal, Greg Montalbano will dive into the details of Digital Therapeutics covering:
• What is driving demand for Digital Therapeutics
• Establishing Digital Therapeutic Regulatory Quality in today's crowded market
• The forms that Digital Therapeutics take on to serve the medical market
2022-07-1317 min MIDI Innovation Vault ™Ep. 1 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotThis series will review the evolution of digital health from isolated remote monitoring technologies ten years ago to an emerging subset of evidence-based, clinically evaluated software products and services. MIDI Principal, Greg Montalbano discusses current and future medical digital healthcare application development that is now moving faster than ever creating an increasingly crowded digital healthcare market. He discusses how the digital therapeutic revolution has generated new healthcare business model opportunities as well as global regulatory, market, and operating model challenges for healthcare and life science companies.2022-06-2911 min
MIDI Innovation Vault ™Ep. 1 Series 7 | Digital Therapeutics: Core Principals, Current Trends & Future SnapshotThis series will review the evolution of digital health from isolated remote monitoring technologies ten years ago to an emerging subset of evidence-based, clinically evaluated software products and services. MIDI Principal, Greg Montalbano discusses current and future medical digital healthcare application development that is now moving faster than ever creating an increasingly crowded digital healthcare market. He discusses how the digital therapeutic revolution has generated new healthcare business model opportunities as well as global regulatory, market, and operating model challenges for healthcare and life science companies.2022-06-2911 min MIDI Innovation Vault ™Ep. 3 Series 6 | MedDevice Supply Chain; Defining & Solving for Supply Chain RiskIn this special episode, MedDevice Supply Chain Paradigm Shift; Defining & Solving for Supply Chain Risk Utilizing MIDI’s De-Risk Method of DFSC, Chris and Pat will share unique and critical knowledge with the medical community.
Chris and Pat step us through the “trials and tribulations” MIDI’s development teams have endured over the last 2 years and reveal unique strategic methods that MIDI has deployed to mitigate our clients' risk in this uncertain world of supply. These approaches are not only critical to our clients' “financial viability” but of course critical to the “health of the general public” as related to be...2022-04-2024 min
MIDI Innovation Vault ™Ep. 3 Series 6 | MedDevice Supply Chain; Defining & Solving for Supply Chain RiskIn this special episode, MedDevice Supply Chain Paradigm Shift; Defining & Solving for Supply Chain Risk Utilizing MIDI’s De-Risk Method of DFSC, Chris and Pat will share unique and critical knowledge with the medical community.
Chris and Pat step us through the “trials and tribulations” MIDI’s development teams have endured over the last 2 years and reveal unique strategic methods that MIDI has deployed to mitigate our clients' risk in this uncertain world of supply. These approaches are not only critical to our clients' “financial viability” but of course critical to the “health of the general public” as related to be...2022-04-2024 min MIDI Innovation Vault ™Ep. 2 Series 6 | Preemptive DFX Practices in MedDevice DevelopmentChris Montalbano, CEO of MIDI walks us through the components of DFX (Design for Excellence). Listen in as Chris "unpacks" each of the DFX components in detail referencing MIDI's methodical yet prudent development approach. Both the functional engineering constraints and the essential business constraints as related to production and costing are considered simultaneously in this development approach.2022-04-0615 min
MIDI Innovation Vault ™Ep. 2 Series 6 | Preemptive DFX Practices in MedDevice DevelopmentChris Montalbano, CEO of MIDI walks us through the components of DFX (Design for Excellence). Listen in as Chris "unpacks" each of the DFX components in detail referencing MIDI's methodical yet prudent development approach. Both the functional engineering constraints and the essential business constraints as related to production and costing are considered simultaneously in this development approach.2022-04-0615 min MIDI Innovation Vault ™Ep. 1 Series 6 | MedTech DFX: Advanced Strategies & Supply Chain Paradigm ShiftThe subject of addressing supply chain issues is quite an important and relevant topic for medical device manufacturers to integrate into their development strategies given the material and component shortages in today's economic environment. The first episode in the new MIDI series, Value of Concurrent Engineering with Inclusion of DFX in MedDevice, Operationalized under ISO-13485, addresses how is it related to an advanced DFX strategy.
Chris Montalbano, CEO of MIDI, helps to define DFX. Additionally, Chris talks about how to operationalize DFX early in the development cycle under a Concurrent Engineering approach as well as what this...2022-03-3011 min
MIDI Innovation Vault ™Ep. 1 Series 6 | MedTech DFX: Advanced Strategies & Supply Chain Paradigm ShiftThe subject of addressing supply chain issues is quite an important and relevant topic for medical device manufacturers to integrate into their development strategies given the material and component shortages in today's economic environment. The first episode in the new MIDI series, Value of Concurrent Engineering with Inclusion of DFX in MedDevice, Operationalized under ISO-13485, addresses how is it related to an advanced DFX strategy.
Chris Montalbano, CEO of MIDI, helps to define DFX. Additionally, Chris talks about how to operationalize DFX early in the development cycle under a Concurrent Engineering approach as well as what this...2022-03-3011 min MIDI Innovation Vault ™Ep. 9 Series 5 | The Ultimate Medical Accelerator & Incubator Lab Executive Summary with Greg MontalbanoIn the last podcast episode of the series, MIDI Medical Product Developments CEO, Gregory Montalbano shares his personal insight as related to what an Accelerator or Incubator can and should be doing for its MedTech Startup entrepreneurial members.
Greg also outlines the “Five Accelerator Model Types” and reveals each accelerator type's own motivations for establishing programs, investing resources and capital as well as the different metrics MedTech Accelerators use for measuring a start-ups success.
2021-12-1526 min
MIDI Innovation Vault ™Ep. 9 Series 5 | The Ultimate Medical Accelerator & Incubator Lab Executive Summary with Greg MontalbanoIn the last podcast episode of the series, MIDI Medical Product Developments CEO, Gregory Montalbano shares his personal insight as related to what an Accelerator or Incubator can and should be doing for its MedTech Startup entrepreneurial members.
Greg also outlines the “Five Accelerator Model Types” and reveals each accelerator type's own motivations for establishing programs, investing resources and capital as well as the different metrics MedTech Accelerators use for measuring a start-ups success.
2021-12-1526 min MIDI Innovation Vault ™Ep. 8 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with TMC Innovation/ JLABS Tom LubyIn this episode, Gregory Montalbano speaks with Dr. Tom Luby who is the Director of Innovation at Texas Medical Center also known as TMC Innovation. In this role, Tom oversees all of the innovation efforts of TMC focused on research, education, and commercialization of novel healthcare solutions. Prior to Tom's role at TMC, he was the head of JLABS at TMC in Houston, Texas.
In this episode, a deep-dive will be taken into the inner-workings of the Houston TMC Innovation and JLABS ecosystem. Greg and Tom discuss the methods of mentoring and support offered to the entrepreneurial...2021-12-0828 min
MIDI Innovation Vault ™Ep. 8 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with TMC Innovation/ JLABS Tom LubyIn this episode, Gregory Montalbano speaks with Dr. Tom Luby who is the Director of Innovation at Texas Medical Center also known as TMC Innovation. In this role, Tom oversees all of the innovation efforts of TMC focused on research, education, and commercialization of novel healthcare solutions. Prior to Tom's role at TMC, he was the head of JLABS at TMC in Houston, Texas.
In this episode, a deep-dive will be taken into the inner-workings of the Houston TMC Innovation and JLABS ecosystem. Greg and Tom discuss the methods of mentoring and support offered to the entrepreneurial...2021-12-0828 min MIDI Innovation Vault ™Ep. 7 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with eHealth Ventures Stephen ShapiroMIDI Medical Product Development’s CEO, Gregory Montalbano speaks with Stephen Shapiro from eHealth Ventures. Steve is the co-founder of eHealth Ventures which is a consortium of world-class health care organizations and investors looking for disruptive digital health companies. After four years eHealth Ventures, located in Tel Aviv Israel, has become one of the world’s top leading global incubators, currently supporting fourteen innovative digital health start-ups and emerging companies. Steve is a pioneer in the computer service, wireless, social media, and digital health sectors with a long history and experience ranging from start-up to successful exit.
On t...2021-12-0848 min
MIDI Innovation Vault ™Ep. 7 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with eHealth Ventures Stephen ShapiroMIDI Medical Product Development’s CEO, Gregory Montalbano speaks with Stephen Shapiro from eHealth Ventures. Steve is the co-founder of eHealth Ventures which is a consortium of world-class health care organizations and investors looking for disruptive digital health companies. After four years eHealth Ventures, located in Tel Aviv Israel, has become one of the world’s top leading global incubators, currently supporting fourteen innovative digital health start-ups and emerging companies. Steve is a pioneer in the computer service, wireless, social media, and digital health sectors with a long history and experience ranging from start-up to successful exit.
On t...2021-12-0848 min MIDI Innovation Vault ™Ep. 6 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with Penn Health-Tech Katie ReutherGregory Montalbano speaks with Katie Reuther who is the Executive Director at Penn Health-Tech located at the University of Pennsylvania in Philadelphia.
Together, Greg and Katie discuss in detail what the Center for Health, Devices, and Technology at the University of Pennsylvania, or Penn Health-Tech for short, and its associated Health-Tech Accelerator is all about.
Greg and Katie discuss the history and inner workings of Penn-Tech Health and the University of Pennsylvania ecosystem partners. Together they discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical, scientific, and biotechnology applications. Katie...2021-12-0129 min
MIDI Innovation Vault ™Ep. 6 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with Penn Health-Tech Katie ReutherGregory Montalbano speaks with Katie Reuther who is the Executive Director at Penn Health-Tech located at the University of Pennsylvania in Philadelphia.
Together, Greg and Katie discuss in detail what the Center for Health, Devices, and Technology at the University of Pennsylvania, or Penn Health-Tech for short, and its associated Health-Tech Accelerator is all about.
Greg and Katie discuss the history and inner workings of Penn-Tech Health and the University of Pennsylvania ecosystem partners. Together they discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical, scientific, and biotechnology applications. Katie...2021-12-0129 min MIDI Innovation Vault ™Ep. 5 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with Center for Biotechnology, Diane FabelMIDI Medical Product Developments CEO, Gregory Montalbano is speaking with Diane Fabel who is the Director of Operations at The Center for Biotechnology located on the campus of Stony Brook University.
On this podcast, Greg and Diane will discuss in detail what The Center for Biotechnology is all about. A deep dive is taken into the inner workings of the Center for Biotechnology ecosystem programs. Together they discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical, scientific, and biotechnology applications. Diane also outlines the various Center for Biotechnology corporate, university, industry as...2021-12-0138 min
MIDI Innovation Vault ™Ep. 5 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with Center for Biotechnology, Diane FabelMIDI Medical Product Developments CEO, Gregory Montalbano is speaking with Diane Fabel who is the Director of Operations at The Center for Biotechnology located on the campus of Stony Brook University.
On this podcast, Greg and Diane will discuss in detail what The Center for Biotechnology is all about. A deep dive is taken into the inner workings of the Center for Biotechnology ecosystem programs. Together they discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical, scientific, and biotechnology applications. Diane also outlines the various Center for Biotechnology corporate, university, industry as...2021-12-0138 min MIDI Innovation Vault ™Ep. 4 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with MedTech Innovator Paul GrandGregory Montalbano speaks with Paul Grand who is the Founder and CEO of MedTech Innovator. Paul runs MedTech Innovator as a stand-alone non-profit which is also the largest accelerator of medical technology in the world.
Together, Greg and Paul discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical device, digital health, and diagnostic applications. Paul will also outline the various MedTech Innovator industry as well as venture partner networks that support their members. Greg and Paul’s discussions will also include what MedTech Innovator looks for in a startup and how they ca...2021-11-1744 min
MIDI Innovation Vault ™Ep. 4 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with MedTech Innovator Paul GrandGregory Montalbano speaks with Paul Grand who is the Founder and CEO of MedTech Innovator. Paul runs MedTech Innovator as a stand-alone non-profit which is also the largest accelerator of medical technology in the world.
Together, Greg and Paul discuss the methods of mentoring and support offered to the entrepreneurial groups spanning medical device, digital health, and diagnostic applications. Paul will also outline the various MedTech Innovator industry as well as venture partner networks that support their members. Greg and Paul’s discussions will also include what MedTech Innovator looks for in a startup and how they ca...2021-11-1744 min MIDI Innovation Vault ™Ep. 3 Series 5 | The Ultimate Medical Development Community with IndieBio Stephen Chambers & Julie WolfMIDI Medical Product Development’s CEO, Gregory Montalbano speaks with Stephen Chambers, Managing Director, and Julie Wolf, Chief Science Officer of the Development Community IndieBio located in heart of New York City.
In this podcast, Stephen and Julie will be discussing in detail what IndieBio NY is all about. Listen for a deep dive into the inner workings of the IndieBio ecosystem. Together, with Greg, they will discuss the methods of mentoring and support offered to the IndieBio entrepreneurial groups spanning medical, scientific, and biotechnology applications. Stephen and Julie will also discuss the various IndieBio partner networks th...2021-11-1734 min
MIDI Innovation Vault ™Ep. 3 Series 5 | The Ultimate Medical Development Community with IndieBio Stephen Chambers & Julie WolfMIDI Medical Product Development’s CEO, Gregory Montalbano speaks with Stephen Chambers, Managing Director, and Julie Wolf, Chief Science Officer of the Development Community IndieBio located in heart of New York City.
In this podcast, Stephen and Julie will be discussing in detail what IndieBio NY is all about. Listen for a deep dive into the inner workings of the IndieBio ecosystem. Together, with Greg, they will discuss the methods of mentoring and support offered to the IndieBio entrepreneurial groups spanning medical, scientific, and biotechnology applications. Stephen and Julie will also discuss the various IndieBio partner networks th...2021-11-1734 min MIDI Innovation Vault ™Ep. 2 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with BioMedX Meghan PinezichOn Episode #2 of MIDI Innovation Vault™ Series; The Deep Dive into Medical & Scientific Accelerators, Incubator Labs and Development Communities, MIDI Medical Product Developments CEO, Gregory Montalbano speaks with Meghan Pinezich who is an Executive Team member of Columbia BioMedX, a biomedical engineering technology accelerator at Columbia University, formerly called the Columbia-Coulter Research Partnership.
Meghan completed her Bachelors in Chemical Engineering at the University of Virginia and Masters in Biomedical Engineering at Columbia University. Currently, Meghan is also a PhD candidate in the laboratory disciplines of Stem Cell and Tissue Engineering.
On today’s podcast, Greg and...2021-11-1027 min
MIDI Innovation Vault ™Ep. 2 Series 5 | The Ultimate Medical Accelerator & Incubator Lab with BioMedX Meghan PinezichOn Episode #2 of MIDI Innovation Vault™ Series; The Deep Dive into Medical & Scientific Accelerators, Incubator Labs and Development Communities, MIDI Medical Product Developments CEO, Gregory Montalbano speaks with Meghan Pinezich who is an Executive Team member of Columbia BioMedX, a biomedical engineering technology accelerator at Columbia University, formerly called the Columbia-Coulter Research Partnership.
Meghan completed her Bachelors in Chemical Engineering at the University of Virginia and Masters in Biomedical Engineering at Columbia University. Currently, Meghan is also a PhD candidate in the laboratory disciplines of Stem Cell and Tissue Engineering.
On today’s podcast, Greg and...2021-11-1027 min MIDI Innovation Vault ™Ep. 1 Series 5 | The Ultimate Medical Accelerator & Incubator Lab Introduction with Greg MontalbanoIn this release of MIDI’s Innovation Vault™ Podcast series principal, Gregory Montalbano hosts deep-dive one-on-one interviews with the Directors of seven top global MedTech and Biotech Accelerators, Incubator Labs, and Development Communities.
Episode 1, introduces and outlines the upcoming series including introductions to each organization's Directors. Greg will then discuss with each organization's Director, various topics involving mentoring and support methods offered to their members, whose technology applications span from medical devices, digital health, diagnostic applications, Biotech, and more.
Additionally, Greg will ask the Directors to share their industry knowledge, experience, insight, and best advice for...2021-11-1020 min
MIDI Innovation Vault ™Ep. 1 Series 5 | The Ultimate Medical Accelerator & Incubator Lab Introduction with Greg MontalbanoIn this release of MIDI’s Innovation Vault™ Podcast series principal, Gregory Montalbano hosts deep-dive one-on-one interviews with the Directors of seven top global MedTech and Biotech Accelerators, Incubator Labs, and Development Communities.
Episode 1, introduces and outlines the upcoming series including introductions to each organization's Directors. Greg will then discuss with each organization's Director, various topics involving mentoring and support methods offered to their members, whose technology applications span from medical devices, digital health, diagnostic applications, Biotech, and more.
Additionally, Greg will ask the Directors to share their industry knowledge, experience, insight, and best advice for...2021-11-1020 min MIDI Innovation Vault ™Ep. 5 Series 4 | Using the Innovation Roadmap to Address Wearable IoMT Challenges, Yielding Innovations - Stop 3In the previous Podcast of this series, Chris Montalbano, CEO of MIDI, provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. He “opened up” the map with us and explained there are three key stops along this journey. We have already reviewed the first 2 stops which did not need to be deployed under the FDA-QSR and ISO-13485 Design Controls and Risk Management. These activities support a programmatic quality feedback loop as well as mitigate risk for Stop #3 of the Innovation Roadmap (Commercialization & Implementation) which does need to be deployed under FD-QSR & ISO 13485, and is greatly detail...2021-07-2121 min
MIDI Innovation Vault ™Ep. 5 Series 4 | Using the Innovation Roadmap to Address Wearable IoMT Challenges, Yielding Innovations - Stop 3In the previous Podcast of this series, Chris Montalbano, CEO of MIDI, provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. He “opened up” the map with us and explained there are three key stops along this journey. We have already reviewed the first 2 stops which did not need to be deployed under the FDA-QSR and ISO-13485 Design Controls and Risk Management. These activities support a programmatic quality feedback loop as well as mitigate risk for Stop #3 of the Innovation Roadmap (Commercialization & Implementation) which does need to be deployed under FD-QSR & ISO 13485, and is greatly detail...2021-07-2121 min MIDI Innovation Vault ™Ep. 4 Series 4 | Using the Innovation Roadmap to Address Wearable IoMT Challenges, Yielding InnovationsIn this episode, Christopher Montalbano, CEO of MIDI steps us through the medical device development Innovation Roadmap. Listen in as Chris helps us pinch-in, or zoom-out on the map, or for those of us who remember using paper maps, we're going to "open up the map" to see the entire scope first and discuss the three key stops along this innovation journey.
Stop 1, Market Exploration, Discovering Opportunities.
Stop 2, Technology Innovations and the R&D Process.
Stop 3, Commercialization and Implementation, which is getting ready for market.
Chris then takes us through a deep dive...2021-07-1432 min
MIDI Innovation Vault ™Ep. 4 Series 4 | Using the Innovation Roadmap to Address Wearable IoMT Challenges, Yielding InnovationsIn this episode, Christopher Montalbano, CEO of MIDI steps us through the medical device development Innovation Roadmap. Listen in as Chris helps us pinch-in, or zoom-out on the map, or for those of us who remember using paper maps, we're going to "open up the map" to see the entire scope first and discuss the three key stops along this innovation journey.
Stop 1, Market Exploration, Discovering Opportunities.
Stop 2, Technology Innovations and the R&D Process.
Stop 3, Commercialization and Implementation, which is getting ready for market.
Chris then takes us through a deep dive...2021-07-1432 min MIDI Innovation Vault ™Ep. 3 Series 4 | Wearable IoMT Physiological Devices; Definitions, Applications, and ChallengesIn the previous episode, Chris Montalbano, CEO of MIDI, provided a detailed deep dive of Wearable IoMT Biosensory Devices. In this episode, Chris will elaborate on what Wearable IoMT Physiological Devices are all about by defining these devices, reviewing their application and then identifying challenges resulting in innovative design solutions.2021-06-3013 min
MIDI Innovation Vault ™Ep. 3 Series 4 | Wearable IoMT Physiological Devices; Definitions, Applications, and ChallengesIn the previous episode, Chris Montalbano, CEO of MIDI, provided a detailed deep dive of Wearable IoMT Biosensory Devices. In this episode, Chris will elaborate on what Wearable IoMT Physiological Devices are all about by defining these devices, reviewing their application and then identifying challenges resulting in innovative design solutions.2021-06-3013 min MIDI Innovation Vault ™Ep. 2 Series 4 | Wearable IoMT Biosensory Devices; Definitions, Applications and ChallengesIn this episode, Chris Montalbano, CEO of MIDI, shares what biosensory devices are all about starting with defining these devices and then looking at the components which make up such devices. In a deeper dive, Chris also provides examples/ applications of these devices seen in everyday life and discusses the development and commercialization challenges tied to innovating solutions.2021-06-2311 min
MIDI Innovation Vault ™Ep. 2 Series 4 | Wearable IoMT Biosensory Devices; Definitions, Applications and ChallengesIn this episode, Chris Montalbano, CEO of MIDI, shares what biosensory devices are all about starting with defining these devices and then looking at the components which make up such devices. In a deeper dive, Chris also provides examples/ applications of these devices seen in everyday life and discusses the development and commercialization challenges tied to innovating solutions.2021-06-2311 min MIDI Innovation Vault ™Ep. 1 Series 4 | What are IoMT Wearables; Use Case Examples, Regulatory Categorization & Defining the IoMT Sensor LandscapeChris Montalbano, CEO of MIDI, discusses what IoMT Wearables are, outlines some of the benefits they provide to the medical community, and then performs a deep dive on device sensor selection. Tune in to hear more about these devices embraced by users as lifestyle devices used in their daily environments. 2021-06-1611 min
MIDI Innovation Vault ™Ep. 1 Series 4 | What are IoMT Wearables; Use Case Examples, Regulatory Categorization & Defining the IoMT Sensor LandscapeChris Montalbano, CEO of MIDI, discusses what IoMT Wearables are, outlines some of the benefits they provide to the medical community, and then performs a deep dive on device sensor selection. Tune in to hear more about these devices embraced by users as lifestyle devices used in their daily environments. 2021-06-1611 min MIDI Innovation Vault ™Ep. 4 Series 3 | Recommended Principals and Practices of Human Factors EngineeringIn Episode 4, titled Recommended Principals and Practices of Human Factors Engineering: The Deep Dive into AAMI HE75 and Methods for Human Factors Usability Research, we discuss and distill down this guidance documents usability research and development methods as related to what medical device developers need to know in order to generate well designed, safe and effective medical devices that will be recognized by the FDA2021-02-1027 min
MIDI Innovation Vault ™Ep. 4 Series 3 | Recommended Principals and Practices of Human Factors EngineeringIn Episode 4, titled Recommended Principals and Practices of Human Factors Engineering: The Deep Dive into AAMI HE75 and Methods for Human Factors Usability Research, we discuss and distill down this guidance documents usability research and development methods as related to what medical device developers need to know in order to generate well designed, safe and effective medical devices that will be recognized by the FDA2021-02-1027 min MIDI Innovation Vault ™Ep. 3 Series 3 | Human Factors Regulatory and Guidance Standards for US SubmissionGreg Montalbano takes our listeners on a deep dive as related to the Human Factors Regulatory Standards and Guidance documents for US submission.
This episode specifically covers topics as related to the various organizations and regulatory bodies as well as standards and guidance documents to utilize for proper usability research and human factors engineering processes during medical device design and development. More specifically, learn more about:
• What organizations have issued United States Regulatory and Guidance documents for Human Factors as related to medical device development.
• The U.S. Standards and Guidelines that apply to certa...2021-02-0333 min
MIDI Innovation Vault ™Ep. 3 Series 3 | Human Factors Regulatory and Guidance Standards for US SubmissionGreg Montalbano takes our listeners on a deep dive as related to the Human Factors Regulatory Standards and Guidance documents for US submission.
This episode specifically covers topics as related to the various organizations and regulatory bodies as well as standards and guidance documents to utilize for proper usability research and human factors engineering processes during medical device design and development. More specifically, learn more about:
• What organizations have issued United States Regulatory and Guidance documents for Human Factors as related to medical device development.
• The U.S. Standards and Guidelines that apply to certa...2021-02-0333 min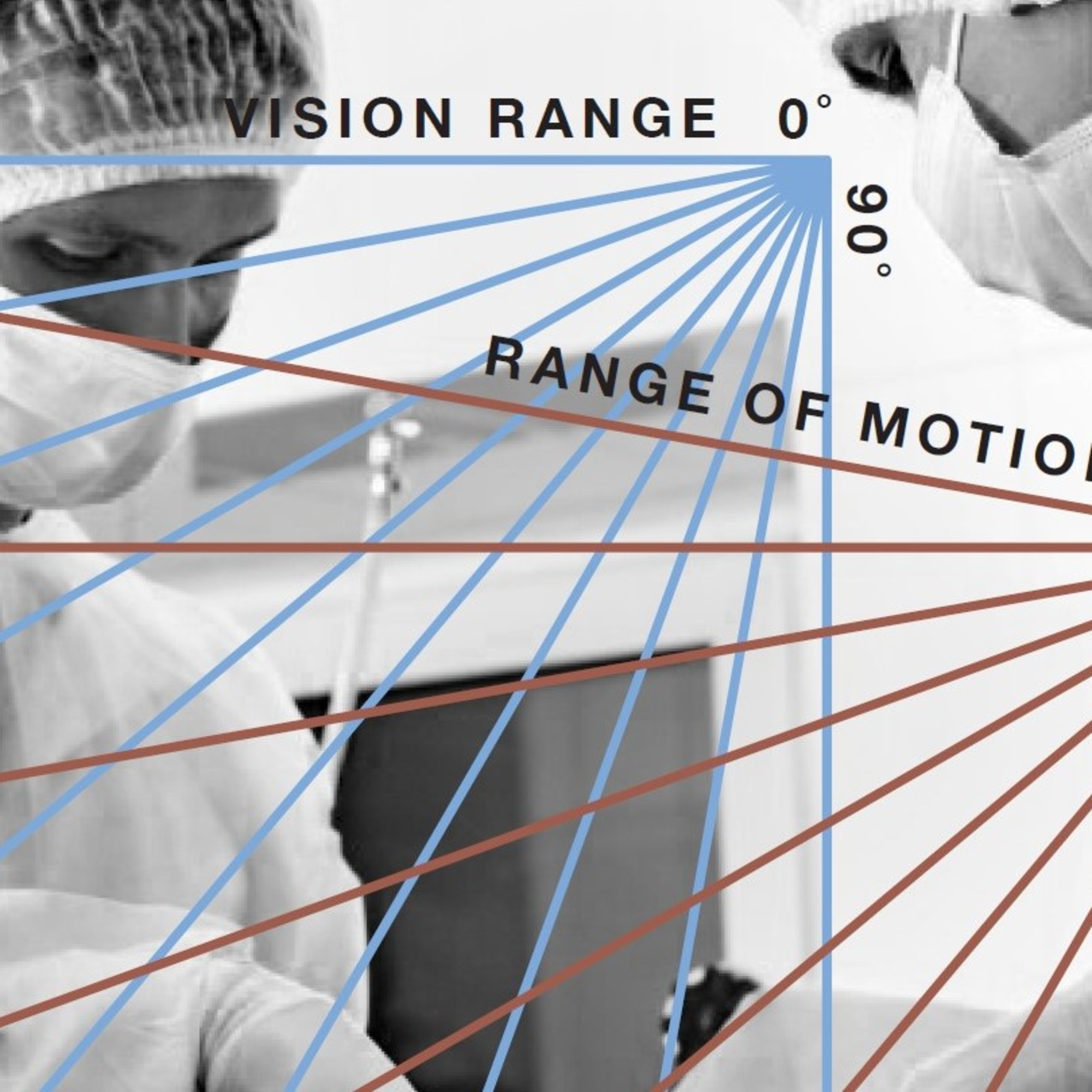 MIDI Innovation Vault ™Ep. 2 Series 3 | Understanding FDA Perspectives on Medical Device Development: Regulatory Requirements, Reasons and Benefits of Human Factors Engineering and Usability ResearchIn this episode, Greg Montalbano, Co-Owner of MIDI takes us on a deep dive into the details as related to the FDA Perspectives on Human Factors and Device Development. We cover topics related to understanding Regulatory Requirements and Methods for Human Factors Usability Testing such as:
• Why is FDA concerned about Human Factors Engineering and Usability
• What is expected of medical device developers from the FDA
• Specific medical device Human Factors Engineering example relative to hazards, risks and the use related causes.2021-01-2712 min
MIDI Innovation Vault ™Ep. 2 Series 3 | Understanding FDA Perspectives on Medical Device Development: Regulatory Requirements, Reasons and Benefits of Human Factors Engineering and Usability ResearchIn this episode, Greg Montalbano, Co-Owner of MIDI takes us on a deep dive into the details as related to the FDA Perspectives on Human Factors and Device Development. We cover topics related to understanding Regulatory Requirements and Methods for Human Factors Usability Testing such as:
• Why is FDA concerned about Human Factors Engineering and Usability
• What is expected of medical device developers from the FDA
• Specific medical device Human Factors Engineering example relative to hazards, risks and the use related causes.2021-01-2712 min MIDI Innovation Vault ™Ep. 1 Series 3 | Human Factors & User Interface Design for Medical Devices: The OverviewThe much anticipated Series 3 podcast is now available!
Join MIDI's principal, Gregory Montalbano, in this series as he shares his deep understanding of the importance, requirements, and methods of Human Factors Engineering (HFE) and Usability Research during the design innovation process of medical devices.2021-01-1924 min
MIDI Innovation Vault ™Ep. 1 Series 3 | Human Factors & User Interface Design for Medical Devices: The OverviewThe much anticipated Series 3 podcast is now available!
Join MIDI's principal, Gregory Montalbano, in this series as he shares his deep understanding of the importance, requirements, and methods of Human Factors Engineering (HFE) and Usability Research during the design innovation process of medical devices.2021-01-1924 min The Honesty Pill PodcastEp. 15 Maren Montalbano: singer, business coach and writer.My guest today is opera singer, business coach, and writer, Maren Montalbano. A graduate of Tufts University and the New England Conservatory of Music, Maren has sung at Carnegie Hall in NYC, Walt Disney Concert Hall in LA, multiple theaters in Europe, and even performed on stage, get this...with the Rolling Stones. Wow. But Maren's career has suffered right along with the rest of us during this past year, but that didn't slow her down. When live performances disappeared during the pandemic, Maren turned her focus to the online world: writing, producing, a...2021-01-1156 min
The Honesty Pill PodcastEp. 15 Maren Montalbano: singer, business coach and writer.My guest today is opera singer, business coach, and writer, Maren Montalbano. A graduate of Tufts University and the New England Conservatory of Music, Maren has sung at Carnegie Hall in NYC, Walt Disney Concert Hall in LA, multiple theaters in Europe, and even performed on stage, get this...with the Rolling Stones. Wow. But Maren's career has suffered right along with the rest of us during this past year, but that didn't slow her down. When live performances disappeared during the pandemic, Maren turned her focus to the online world: writing, producing, a...2021-01-1156 min MIDI Innovation Vault ™Ep. 5 Series 2 | Following the INNOVATION ROADMAP™: Stop 3: PART-2The FINAL episode in the series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In this episode, we're joined by Andrew Martin, Vice President of MIDI. So far in this series, we have covered class identification, planning, requirements, and our MATRIX-ALM design control tool. Now, in this episode, we cover RISK (ISO 14971) and utilizing MATRIX to document RISK. Listen in as Andrew dives into everything from Risk analysis to production and post-production activities.2020-09-1709 min
MIDI Innovation Vault ™Ep. 5 Series 2 | Following the INNOVATION ROADMAP™: Stop 3: PART-2The FINAL episode in the series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In this episode, we're joined by Andrew Martin, Vice President of MIDI. So far in this series, we have covered class identification, planning, requirements, and our MATRIX-ALM design control tool. Now, in this episode, we cover RISK (ISO 14971) and utilizing MATRIX to document RISK. Listen in as Andrew dives into everything from Risk analysis to production and post-production activities.2020-09-1709 min MIDI Innovation Vault ™Ep. 4 Series 2 | Following the INNOVATION ROADMAP™: Stop 3: PART-1So far in the series, we have provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained there are three key stops along this journey. We have already reviewed the first TWO stops which DID NOT need to be deployed under the FDA-QSR and ISO-13485 Design Controls and Risk Management. These first TWO stops allowed us to explore the market, identify opportunities, and then perform exploratory technology investigations and interactive MVP breadboarding in support of and refinement of these opportunities.
In this episode, we're joined by our expert...2020-09-1017 min
MIDI Innovation Vault ™Ep. 4 Series 2 | Following the INNOVATION ROADMAP™: Stop 3: PART-1So far in the series, we have provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained there are three key stops along this journey. We have already reviewed the first TWO stops which DID NOT need to be deployed under the FDA-QSR and ISO-13485 Design Controls and Risk Management. These first TWO stops allowed us to explore the market, identify opportunities, and then perform exploratory technology investigations and interactive MVP breadboarding in support of and refinement of these opportunities.
In this episode, we're joined by our expert...2020-09-1017 min MIDI Innovation Vault ™Ep. 3 Series 2 | The INNOVATION ROADMAP™: Stop 2: Technology Innovation and the R&D ProcessListen to the newest episode in our podcast series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In our previous episode, Chris Montalbano provided an overview of the INNOVATION ROADMAP™ as applied to medical device development in addition to describing the 1st Stop which was Market Exploration & Discovering Opportunities. In this new episode, Principal and Chief Creative Officer, Gregory Montalbano discusses the INNOVATION ROADMAP™ 2nd Stop, which is Technology Innovation and the R&D Process.
As discussed in prior episodes, the FDA-QSR & ISO 13485 guidance recognizes both Stop 1 Market Exploration & Discovering Opportunities as well as Sto...2020-07-2911 min
MIDI Innovation Vault ™Ep. 3 Series 2 | The INNOVATION ROADMAP™: Stop 2: Technology Innovation and the R&D ProcessListen to the newest episode in our podcast series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™."
In our previous episode, Chris Montalbano provided an overview of the INNOVATION ROADMAP™ as applied to medical device development in addition to describing the 1st Stop which was Market Exploration & Discovering Opportunities. In this new episode, Principal and Chief Creative Officer, Gregory Montalbano discusses the INNOVATION ROADMAP™ 2nd Stop, which is Technology Innovation and the R&D Process.
As discussed in prior episodes, the FDA-QSR & ISO 13485 guidance recognizes both Stop 1 Market Exploration & Discovering Opportunities as well as Sto...2020-07-2911 min MIDI Innovation Vault ™Ep. 2 Series 2 | Following the INNOVATION ROADMAP™: Stop -1, Market Exploration: Discovering OpportunitiesThe first episode of this series provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained the three key stops along this journey.
Now, we dive into the 1st Stop which is Market Exploration & Discovering Opportunities. The FDA-QSR & ISO-13485 guidance recognizes this as a quintessential activity for any business to perform, yet they make it known that their regulatory controls such as Design Controls & Risk Management do not have to be performed at this point. They understand the importance of a company needing to explore opportunities first.
L...2020-07-2210 min
MIDI Innovation Vault ™Ep. 2 Series 2 | Following the INNOVATION ROADMAP™: Stop -1, Market Exploration: Discovering OpportunitiesThe first episode of this series provided an overview of the INNOVATION ROADMAP™ as applied to Medical Device Development. We “opened up” the map and explained the three key stops along this journey.
Now, we dive into the 1st Stop which is Market Exploration & Discovering Opportunities. The FDA-QSR & ISO-13485 guidance recognizes this as a quintessential activity for any business to perform, yet they make it known that their regulatory controls such as Design Controls & Risk Management do not have to be performed at this point. They understand the importance of a company needing to explore opportunities first.
L...2020-07-2210 min MIDI Innovation Vault ™Ep 1. Series 2 | Paradigm Perception Shift in Medical Device Development: the INNOVATION ROADMAP™Episode 1 in our new series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™ " is now available.
Listen to this first episode as we prepare for an exploration "Explaining the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485" with Co-Owner of MIDI Medical Product Development, Chris Montalbano.
In this episode, Chris outlines what's behind the MIDI expert DevelopmentDNA™ approach. He explains that the common perception in the industry is that the Medical Regulatory Design Controls and Risk management (meaning FDA-QSR & ISO-13485) are often viewed as a mandate, a process that w...2020-07-1509 min
MIDI Innovation Vault ™Ep 1. Series 2 | Paradigm Perception Shift in Medical Device Development: the INNOVATION ROADMAP™Episode 1 in our new series, "PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™ " is now available.
Listen to this first episode as we prepare for an exploration "Explaining the INNOVATION ROADMAP™: Device Innovation as a Result of Embracing Regulatory Controls: FDA-QSR & ISO-13485" with Co-Owner of MIDI Medical Product Development, Chris Montalbano.
In this episode, Chris outlines what's behind the MIDI expert DevelopmentDNA™ approach. He explains that the common perception in the industry is that the Medical Regulatory Design Controls and Risk management (meaning FDA-QSR & ISO-13485) are often viewed as a mandate, a process that w...2020-07-1509 min MIDI Innovation Vault ™Ep 5. | Paradigm Shift Required: Technological Approaches to Infection PreventionThis wrap up episode features special guest Christian Davis, VP of Product Development & Technology at Seal Shield. Chris Montalbano, MIDI Principal and Christian discuss Technological Approaches to infection prevention.
In the 4 previous episodes, we have detailed how current methods of HAI control in hospital environments can be applied to environments we encounter in our everyday lives which would help in the fight against COVID-19. We then explored what areas in both these environments would require protection and how the technologies might manifest themselves in the way of semi-automated or automated devices, providing for a passive user ceremony.2020-05-2812 min
MIDI Innovation Vault ™Ep 5. | Paradigm Shift Required: Technological Approaches to Infection PreventionThis wrap up episode features special guest Christian Davis, VP of Product Development & Technology at Seal Shield. Chris Montalbano, MIDI Principal and Christian discuss Technological Approaches to infection prevention.
In the 4 previous episodes, we have detailed how current methods of HAI control in hospital environments can be applied to environments we encounter in our everyday lives which would help in the fight against COVID-19. We then explored what areas in both these environments would require protection and how the technologies might manifest themselves in the way of semi-automated or automated devices, providing for a passive user ceremony.2020-05-2812 min MIDI Innovation Vault ™Ep 4. | Current and Future Industry and Technology Applications for Infection Prevention Technology & DevelopmentPart four of Paradigm Shift Required; Current and Future Industry and Technology Applications for Infection Prevention Technology & Development with host Matt Boutwell and Chief Creation Officer, Greg Montalbano of MIDI Product Development. 2020-05-1916 min
MIDI Innovation Vault ™Ep 4. | Current and Future Industry and Technology Applications for Infection Prevention Technology & DevelopmentPart four of Paradigm Shift Required; Current and Future Industry and Technology Applications for Infection Prevention Technology & Development with host Matt Boutwell and Chief Creation Officer, Greg Montalbano of MIDI Product Development. 2020-05-1916 min MIDI Innovation Vault ™Ep 3. | Automation of Infection Prevention Technology & DevelopmentGreg Montalbano, the Chief Creation Officer for MIDI Medical Product Development, joins host Matt Boutwell for Episode 3 in the Innovation Vault™. In episode 3 of this 5 part series, we'll hear Greg’s insight and experience on the subject of medical device market innovation and technology application for automation and semi-automation of infection prevention and detection procedures.
Listen as Greg goes into great detail to give you insight on what is happening today in the world of infection prevention. 2020-05-1219 min
MIDI Innovation Vault ™Ep 3. | Automation of Infection Prevention Technology & DevelopmentGreg Montalbano, the Chief Creation Officer for MIDI Medical Product Development, joins host Matt Boutwell for Episode 3 in the Innovation Vault™. In episode 3 of this 5 part series, we'll hear Greg’s insight and experience on the subject of medical device market innovation and technology application for automation and semi-automation of infection prevention and detection procedures.
Listen as Greg goes into great detail to give you insight on what is happening today in the world of infection prevention. 2020-05-1219 min MIDI Innovation Vault ™Ep 2. Infection Prevention - Environments to Protect: Then & NowPart one of this series, Chris Montalbano, Principal of MIDI Product Development joined Host Matt Boutwell as they focused on the perceptions and approaches to infection prevention, then and now. In part two, we're diving into the environments.
In the past, a central area of concern was in protecting hospital patients with a compromised immune system leaving them susceptible to hospital-acquired infections.
Today, COVID-19 has changed how we work, travel, and shop for the foreseeable future. These densely populated spaces need to be protected and addressed. Additionally, this virus has revealed multiple flaws in the...2020-05-0412 min
MIDI Innovation Vault ™Ep 2. Infection Prevention - Environments to Protect: Then & NowPart one of this series, Chris Montalbano, Principal of MIDI Product Development joined Host Matt Boutwell as they focused on the perceptions and approaches to infection prevention, then and now. In part two, we're diving into the environments.
In the past, a central area of concern was in protecting hospital patients with a compromised immune system leaving them susceptible to hospital-acquired infections.
Today, COVID-19 has changed how we work, travel, and shop for the foreseeable future. These densely populated spaces need to be protected and addressed. Additionally, this virus has revealed multiple flaws in the...2020-05-0412 min MIDI Innovation Vault ™Ep 1. Infection Prevention - Perceptions and Approaches: Then & NowJoin Chris Montalbano, Principal MIDI Medical Product Development, and Host Matt Boutwell, as they discuss infection prevention within healthcare environments.
Prior to the pandemic, one of MIDI's Medical Device Development areas of focus was on the creation of infection prevention solutions in the healthcare setting. Chris states that "We view the most recent events as a call to action at a more rapid and broader scale."
Listen as Matt & Chris talk about the changes in risks, the perceptions, and what is really going to help mitigate loss of life related to infections.2020-05-0412 min
MIDI Innovation Vault ™Ep 1. Infection Prevention - Perceptions and Approaches: Then & NowJoin Chris Montalbano, Principal MIDI Medical Product Development, and Host Matt Boutwell, as they discuss infection prevention within healthcare environments.
Prior to the pandemic, one of MIDI's Medical Device Development areas of focus was on the creation of infection prevention solutions in the healthcare setting. Chris states that "We view the most recent events as a call to action at a more rapid and broader scale."
Listen as Matt & Chris talk about the changes in risks, the perceptions, and what is really going to help mitigate loss of life related to infections.2020-05-0412 min Last WordAndrea Camilleri, Johnny Clegg OBE, OIS, Christopher Kraft, Paul Krassner, Professor Rolf GehlhaarPictured: Andrea CamilleriMatthew Bannister on Andrea Camilleri, the Sicilian writer who created the Inspector Montalbano novels. His literary success began in his late sixties.Johnny Clegg, the white South African singer and guitarist who confronted the apartheid government by embracing Zulu music and culture. Christopher Kraft, the founder of mission control for the NASA space programme.Paul Krassner, the controversial American satirist who coined the word "Yippie".Professor Rolf Gehlhaar, the electronic music composer and technician who co-founded the Paraorchestra.Interviewed guest: John Hooper
...2019-07-2628 min
Last WordAndrea Camilleri, Johnny Clegg OBE, OIS, Christopher Kraft, Paul Krassner, Professor Rolf GehlhaarPictured: Andrea CamilleriMatthew Bannister on Andrea Camilleri, the Sicilian writer who created the Inspector Montalbano novels. His literary success began in his late sixties.Johnny Clegg, the white South African singer and guitarist who confronted the apartheid government by embracing Zulu music and culture. Christopher Kraft, the founder of mission control for the NASA space programme.Paul Krassner, the controversial American satirist who coined the word "Yippie".Professor Rolf Gehlhaar, the electronic music composer and technician who co-founded the Paraorchestra.Interviewed guest: John Hooper
...2019-07-2628 min Get BookedThere's Always a DukeAmanda and Jenn discuss food memoirs, satires, books to build empathy, and more on this week’s episode of Get Booked.This episode is sponsored by Madison Reed and Comic Bento.This content contains affiliate links. When you buy through these links, we may earn an affiliate commission.For listener feedback and questions, as well as a complete list of books discussed in this episode, visit our website.Books DiscussedThe Perfect Storm by Sebastian JungerSalvage the Bones by Jesmyn WardIsa...2016-12-0659 min
Get BookedThere's Always a DukeAmanda and Jenn discuss food memoirs, satires, books to build empathy, and more on this week’s episode of Get Booked.This episode is sponsored by Madison Reed and Comic Bento.This content contains affiliate links. When you buy through these links, we may earn an affiliate commission.For listener feedback and questions, as well as a complete list of books discussed in this episode, visit our website.Books DiscussedThe Perfect Storm by Sebastian JungerSalvage the Bones by Jesmyn WardIsa...2016-12-0659 min